Vitamin B12 (Cyanocobalamin)
Vitamin B12 is the most potent vitamin and is also called anti-pernicious anemia vitamin. It is the factor present in the liver extract which is responsible for the beneficial effects in pernicious anemia. It was previously described as extrinsic or food factor of Castle.
Occurrence of Vitamin B12
This vitamin is not present in Plants. Liver and kidneys are the chief sources but it also occurs in milk, meat, eggs and fish. Another source in man may be the intestinal bacteria but it is quite variable.
Chemistry of Vitamin B12
Vitamin B12 is the only naturally occurring organic compound which contains cobalt (35%). It is water soluble, deep red, tasteless, crystalline compound with a molecular weight of 1355 and is hence stable at neutral pH but not at alkaline pH.
The chemical Structure of vitamin B12 is given below: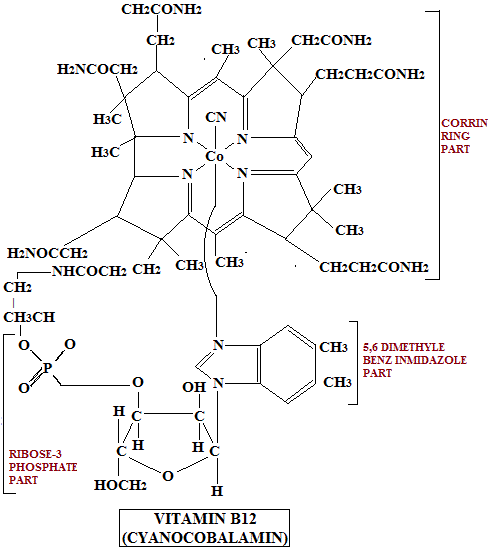
The salient features of the structure of Vitamin B12 are given below:
- There are many closely related compounds having B12 activity. All are cobalamins and contain in their molecules a portion called corrin ring which to a large extent resembles the tetrapyrrole ring structure of porphyrins.
- A single cobalt atom having one positive charge is present in the center of this ring. The Co atom is attached to all the four N atoms of corrin ring in the same way as Fe++ is attached to 5,6, dimethylbenzimidazole ribose which is attached to the side chain on ring IV through Phosphate and aminopropanol.
- The positive charge on Co atom is balanced by one of the several groups which give rise to more than one type of vitamin B12. These different types of vitamin B12 are given below along with their characteristic groups attached to the Co atom
B12 : Cyanocobalamin: It has CN. It is the commonly used form of the vitamin B12
B12-a : hydroxocobalamin: It has OH. It is claimed to be retained in the body
B12-b : aquocobalamin: It has H2O
B12-c : nitrocobalamin: It has NO2
Two more types of Vitamin B12 are chlorocobalamin and thiocyanatocobalamin.
 Biochemical Role of Vitamin B12 (Cyanocobalamin)
Biochemical Role of Vitamin B12 (Cyanocobalamin)
The different types of vitamin B12 described above are actually artifacts and the various groups attached to the Co atom are incorporated in the structure during isolation processes. In the body all types of Vitamin B12 are converted to coenzymes B12 which are called cobamides. These coenzymes do not contain –CN or other groups mentioned above but contain one of the two groups attached to the Co atom.
- Adenine nucleoside (5-deoxyadenosine). There are three coenzymes of this type which differ from each other in respect of benzimidazole portion of the vitamin B12molecule. One of these has 5,6,dimethylbenzimidazole, the second has benzimidazole and the third has adenyl group; they are respectively called DBC, BC and AC. In human liver DBC is the most predominant coenzyme B12.
- Methyle group: A coenzyme B12 containing a methyl group attached to the Co atom has also been discovered.
Reactions in which the coenzyme B12 are involved are given below:
1. Transmethylation reaction in which thymine, methionine and choline are synthesized.
In this case the coenzyme B12 acts as a hydrogen transfer. Deoxyribonucleotides are utilized in the synthesis of DNA.
In vitamin B12 deficiency methylamalonic acid accumulates and is excreted in urine in increased amounts.
Other reactions in which this vitamin has been shown to take part are the conversion of folic acid to its active coenzyme forms, metabolism of several amino acids, choline and cholesterol, porphyrin biosynthesis and activation of amino acids for protein synthesis.




